The application for reference listed drug selection of glycerol phenylbutyrate oral liquid (trade name: RAVICTI®) was officially approved by the National Medical Products Administration on August 16, 2021. It is approved to be included in the Catalog of Reference Listed Drugs for Generic Drugs (Batch 44), with serial No. 44-44[1]. RAVICTI® has also been approved to be reference listed drug in the United States, and is included in the Orange Book[2] of the US FDA. The complete and sufficient safety and efficacy data of RAVICTI® provide the basis for its marketing in the United States and Europe.
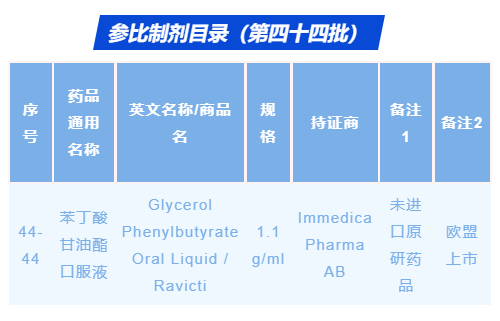
Website: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20210818084801156.html
RAVICTI® is mainly used for the long-term treatment of patients with urea cycle disorders (UCDs). UCDs is a rare and life-threatening disease. At present, there is no effective therapeutic drugs for this disease in China. Among the indications of RAVICTI® approved by the European Medicines Agency (EMA), 4 subtypes (ASS, ARG, OTC and HHH) have been included in the First Batch of Rare Diseases [3] jointly formulated by the National Health Commission and other five departments in 2018, and included in the Guidelines for the Diagnosis and Treatment of Rare Diseases (2019 Edition) [4] issued by the General Office of the National Health Commission.
Being identified as a reference listed drug by the National Medical Products Administration is the first important milestone in the marketing process of RAVICTI® in China, which plays a positive role in promoting the registration and marketing plan of RAVICTI® in China. Hong Kong Winhealth Pharma Group will actively communicate with the Center for Drug Evaluation of the National Medical Products Administration in the near future and make joint efforts to accelerate the pace of marketing evaluation and approval, so as to make the drug available in the market as soon as possible, and enable patients to receive timely rescue and effective treatment. At the same time, Hong Kong Winhealth Pharma Group will also rationally plan and arrange the application and marketing of RAVICTI® in neighboring countries, so as to contribute to improving the accessibility of patients in China and neighboring countries to global innovative drugs.
As a China-based and global innovative biomedical company, Hong Kong Winhealth Pharma Group has been committed to providing novel breakthrough therapies to patients with rare diseases and other unmet medical needs, and insists on introducing more original and innovative drugs from around the world to bring more diversified treatment regimes and recovery opportunities to Chinese patients.
About Glycerol Phenylbutyrate Oral Liquid
Glycerol phenylbutyrate oral liquid (trade name: RAVICTI®) is an innovator drug registered in Europe by Immedica Pharma AB, Sweden, which is used for the treatment of urea cycle disorders (UCDs). The marketing application of this drug was submitted to EMA via centralized review procedures on June 2, 2014, and was approved for marketing on November 27, 2015.
About Urea Cycle Disorders (UCDs)
The urea cycle disorders (UCDs) comprise diseases presenting with hyper ammoniemia that arise in either neonatal period or later. Congenital defects of the enzymes or transporters of the urea cycle cause the disease. This cycle utilizes five enzymes, carbamoylphosphate synthetase 1 and ornithine transcarbamylase are present in the mitochondrial matrix, where as the others (argininosuccinate synthetase, argininosuccinate lyase and arginase 1) are present in the cytoplasm. In addition, N-acetylglutamate synthase and at least two transporter proteins are essential to urea cycle function. Severity and age of onset depend on residual enzyme or transporter function and are related to the respective gene mutations. UCDs symptoms correlate to the ammonia level in the body. [5]
About Winhealth Pharma
Hong Kong Winhealth Pharma Group is a China-based, global innovative biomedical company founded in 2006, providing novel breakthrough therapies to patients with rare diseases and other unmet medical needs. The Group has established long-term strategic partnership with dozens of world-leading biotechnology companies, including Immedica, Roche, Pfizer, Kyowa Kirin, Shionogi, Cumberland Pharmaceutical, and Daiichi-Sankyo. It has built a unique, balanced and diversified portfolio with numerous innovative rare disease and specialty products at commercial and late clinical stages, some with blockbuster potential, and will continuously look to bring in more innovative therapies from the globe.
References:
[1]: Announcement of the National Medical Products Administration on Issuing the Catalog of Reference Listed Drug for Generic Drugs (Batch 44) ([2021] No. 61)
[2]: U.S. Food and Drug Administration. Orange Book [EB]
.https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm, August 2021/August 23th 2021.
[3]: Notice on the Publication of the First Batch of Rare Diseases (issued by the National Health Commission of the PRC, [2018] No. 10)
[4]: Notice of the General Office of the National Health Commission on Issuing the Guidelines for the Diagnosis and Treatment of Rare Diseases (2019 Edition) (Medical Letter of National Health Commission, [2019] No. 198)
[5]: J Hum Genet. 2019; 64 (9): 833-847